Central Connecticut Behavioral Health
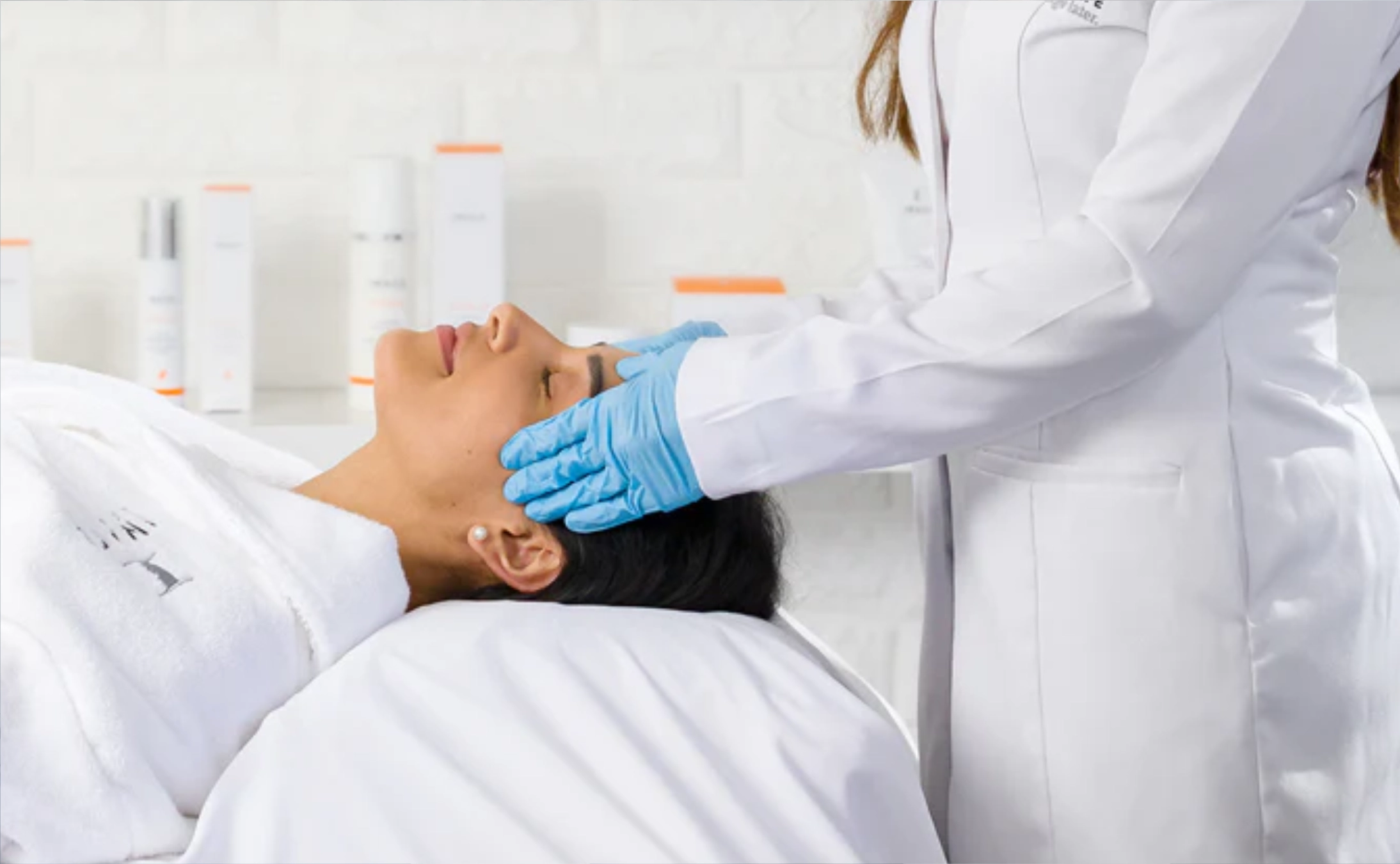
Central Connecticut Behavioral Health (CCBH) provides nasal spray therapy to depressed adults who have not improved with the use of ordinary medicines. We administer Spravato (esketamine) Nasal Spray Treatment, a fast-acting spray that affects other chemicals in the brain than conventional antidepressants.
When you visit, we take you through the process step by step. The session takes approximately two hours, and we observe your reaction to make you feel safe and comfortable. It is not uncommon for many patients to begin to feel better after only a few treatments. This is one of the options that we have in our mission to provide you with hope when other treatments have failed.
Central Connecticut Behavioral Health
Central Connecticut Behavioral Health (CCBH) provides adults who deal with treatment-resistant depression with nasal spray therapy. This is a depression that standard drugs cannot help. The machine-made Spravato (esketamine) Nasal Spray Treatment is rapid-acting; its nasal spray therapy raises spirits, but it impacts another brain pathway than conventional antidepressants. This treatment is offered in a setting of a peaceful and caring clinic area where our medical specialists will closely follow your health and safety.
The duration of a single session is approximately two hours. We will begin with measuring your vital signs and ensuring that you are ready. You will lie in a quiet room after applying the nasal spray, where we will observe your reaction. We hang around with you throughout in order to feel that you are being supported and safe. In the initial periods, most individuals come to it two times per week, but eventually, they come less frequently. Most of our patients start seeing positive changes even after a couple of sessions. Central Connecticut Behavioral Health (CCBH) will assist you and make you feel better.

Our Procedure
Initial Evaluation
Personalized Treatment Plan
Pre-Treatment Preparation
In-Clinic Administration
Ongoing Monitoring
Post-Treatment Support

How Does
At Central Connecticut Behavioral Health (CCBH), we provide Spravato (esketamine), an FDA-approved depression medicine used by adults who have not found themselves any better, even after taking routine antidepressants. Our clinic administers a nasal spray of it. Spravato is an alternative medication that affects some chemicals in the brain that raise mood and lower the feeling of sadness. It can provide relief much quicker than conventional medications, and most of our patients are feeling much better after only a few visits.
We use esketamine for mood disorders in cases where other issues have not had an effect. It attacks those parts of the brain that are associated with depression and helps to bring some chemical harmony. Our staff supervises every session to ensure you remain safe and supported. We are here to walk you through this and assist you in finding hope and healing, even if you’ve been struggling for a long time.
Why Choose Our

FDA-Approved and Effective
We offer an antidepressant approved by the FDA called Spravato (esketamine). It does not work in the same way regular antidepressants do, and can be an immediate relief experience for those who have not been responding to other medications.

Safe and Supervised Care
The medical supervision is carried out in our clinic regarding all the treatments. During each session, we take a record of your mood, blood pressure, and side effects. Our trained personnel keep you and your well-being safe, comfy, and secure over time.

Personalized Treatment Plans
The medical supervision is carried out in our clinic regarding all the treatments. During each session, we take a record of your mood, blood pressure, and side effects. Our trained personnel keep you and your well-being safe, comfy and secure over time.

Experienced Mental Health Team
Our mental health professionals have acquired skills to administer esketamine in mood disorders at our facility. We have a rich history of helping patients who encounter depression that is hard to cure, and we will be with you till the end of your recovery.
Comfortable In-Clinic Environment
Our clinic has a relaxed and quiet environment in which treatment is offered. Each of our sessions would involve being in a separate room where we would study while you relax. We make our best efforts to make you safe and secure.
Continued Emotional Support
We follow up with you, provide medication management, and continue to give you therapy even when you are done with a spray session of Spravato. Our mission is to heal you in a lifetime, to help you always be well, and to live your life with hope.
Contact Us for
Spravato (esketamine) Nasal Spray Treatment
In case you or your loved one is struggling with depression that has not improved with regular medicines, we are available. In Central Connecticut Behavioral Health (CCBH), we provide Spravato (esketamine) nasal spray medication in an accepting, non-threatening, and calm environment.
We have a team that is well-versed in all the steps involved, which will guide you through each process, right from the first assessment to all the treatment sessions. We are here to answer all of your questions, to explain to you how the treatment is to occur, and to ensure that you feel confident. Contact us at Central Connecticut Behavioral Health (CCBH), or visit our clinic. We will be glad to accept your first appointment and assist you in discussing your needs in detail. We know that depression is a severe condition, and we will do our best to help you eliminate it and give you hope.